Molecular Switches
Since 2009, we have been interested in two kinds of MMS :
Both systems can be reversibly switched between two electronic configurations under the application of external stimuli (light, temperature, pressure, electric field, etc.).
This switching between two electronic configurations are accompanied by changes in the physical properties (magnetic, optical, dielectric,...)
These changes can be drastic in particular for Fe(II) SCO and cyanide-bridged Fe-Co CT complexes.
To learn more about our work, see below
Few words about our work on Charge Transfer switchable complexes

Samantha
Harrison
Marketing Executive
I'm a paragraph. Click here to add your own text and edit me. It’s easy. Just click “Edit Text” or double click me.

SQUARES
We can start the story here, in 2009, with a difficult synthesis of a tridentate facial ligand that we obtained at the mg scale...
Luckily the first step in that synthesis was the preparation of a bidentate ligand, bis(N-methyl-imidazolyl)ketone (named bik), that turns out to be very useful (di-imine N2 donor) ligand for obtaining MMS...
check for in publication list
CUBES
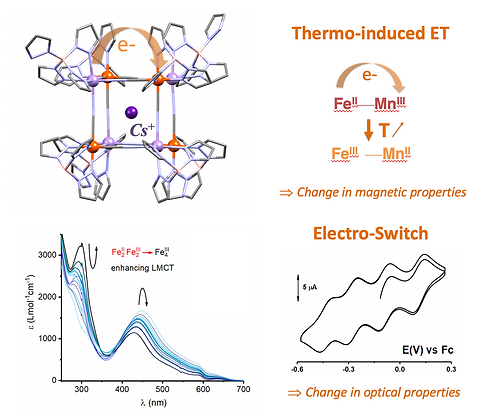
redox switch + magnetic switch
Since 2016, we have focused our research on MMS cubic molecules. These octametallic M4M'4 cubes are not only beautiful MMS but they also exhibit a remarkable redox behaviour with up to nine reversibly accessible redox states...
check for in publication list

Samantha
Harrison
Marketing Executive
I'm a paragraph. Click here to add your own text and edit me. It’s easy. Just click “Edit Text” or double click me.

Few words about our work on SCO complexes
Monometallic SCO complexes
The R-bik ligands are beta-diimine ligands, which are reminiscent of the well known alpha-diiimine ligands: 2,2'-bipyridine or 1,10-phenanthroline
... a noticeable difference though : the R-bik is flexible and it bents ! The dihedral angle between the two Imidazolyl groups can vary from 0 to 20° in our complexes, which impacts the ligand field.
When planar, the ligand field is higher because of a stronger p-acid character, as the central keto group participate to an extended p-systems. However, in the bent form the ligand field decreases,...
In our study, the ligand field can also be modulated by ligand substitution on the Im rings, but to a weaker extent.

Polymetallic SCO complexes
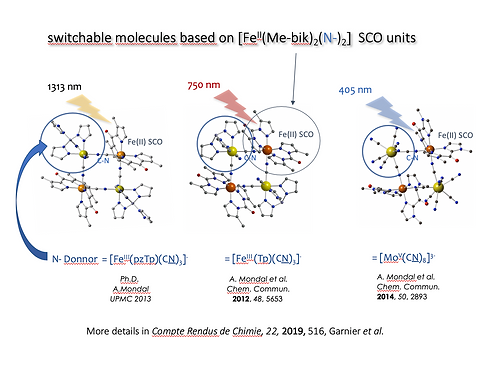
The CoN6 coordination site, found in the FeCo squares shown above, ressembles some typical surrounding of Fe(II) SCO complexes (e.g. Fe(phen)2(NCS)2). Thus, in many of our compounds, replacing the Co(II) ion by Fe(II) leads to interesting SCO behaviour.
These complexes often show LIESST effect : the diamagnetic low-spin state is converted into a metastable paramagnetic high-spin state at low T ( = photomagnetism). We observed that the nature of the N-donor atom radically change the efficient wavelength promoting the LIESST effect
check for in publication list